acoramidis


TTR stabilizer for transthyretin amyloidosis (ATTR)
Acoramidis is an investigational, oral drug designed to potently stabilize transthyretin (TTR). In 2023, BridgeBio announced positive results from ATTRibute-CM, a Phase 3 study of acoramidis in ATTR cardiomyopathy (ATTR-CM). For patients with ATTR-CM, TTR stabilization offers the chance to both preserve the cardio-protective benefits of TTR and address the root cause of disease. The U.S. FDA has accepted the New Drug Application (NDA) and the European Medicines Agency (EMA) accepted the Marketing Authorization Application with additional global regulatory submissions planned.
The safety and efficacy of acoramidis have not been fully established. There is no guarantee that acoramidis will receive health authority approval or become commercially available in any country for the uses being investigated.
acoramidis facts
disease
ATTR-CM
genetic source
TTR (transthyretin)
clinical phase
Phase 3
modality
small molecule
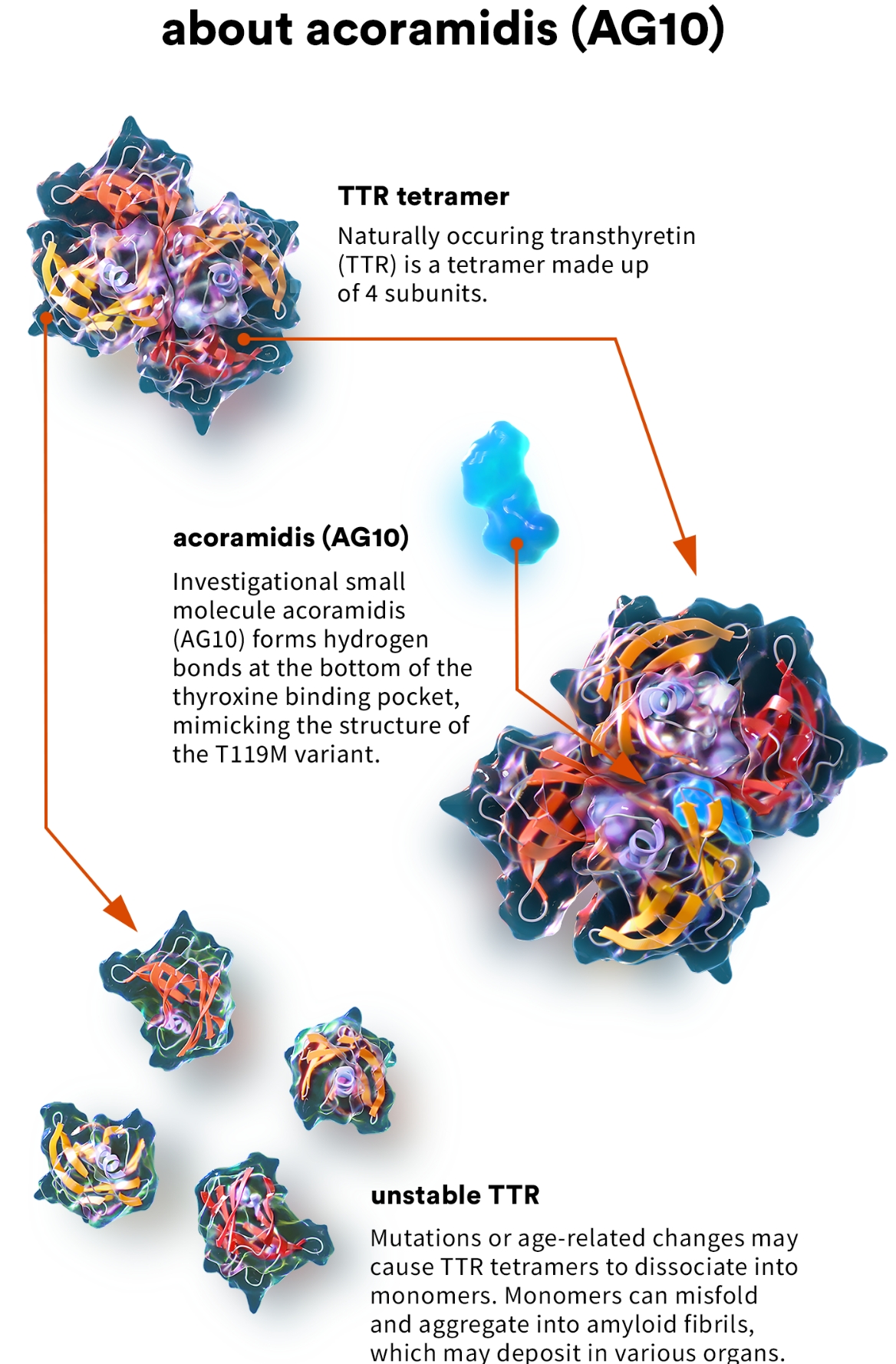
our approach
Acoramidis is a next-generation TTR stabilizer designed by researchers at Stanford University to mimic the structure and stabilizing effect of the naturally protective T119M variant. T119M is known to protect individuals from developing ATTR-CM due to the formation of hydrogen bonds at the center of the molecule that stabilize the protein approximately 40-fold beyond the native structure.1
In an analysis of an open-label extension of a Phase 3 study of TTR-stabilizing medicine in patients with ATTR-CM, it was found that a greater level of TTR stabilization led to improved clinical benefit in ATTR-CM patients.2
related news
presentations & publications
References
- Hammarstrom, P. et al. PNAS 2002, 99:16427 – 16432.
- Judge et al. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. JAMA Cardiol. 2019;74(3)285-295. doi.org/10.1016/j.jacc.2019.03.012.