QED and Parent Company BridgeBio Announce Preclinical Data Supporting Tolerability and Activity of Low-dose Infigratinib in Treating Achondroplasia
Data presented at the American Society of Human Genetics (ASHG) 2019 Annual Meeting
- Infigratinib showed improvements in nine measures of bone development in the Fgfr3Y367C/+ mouse model of achondroplasia at a low dose
- Observational study (The PROPEL Trial) is currently enrolling children age 2.5-10 years and planned submission of an investigational new drug application remains on track for 2020
SAN FRANCISCO – October 17, 2019 – QED Therapeutics, Inc. and parent company BridgeBio Pharma, Inc. (NASDAQ: BBIO) announced today the presentation of preclinical data supporting the potential of low-dose infigratinib for the treatment of achondroplasia. The data were presented during the ASHG 2019 Annual Meeting in a poster entitled “Low dose, daily or intermittent administration of infigratinib (BGJ398), a selective FGFR inhibitor, as treatment for achondroplasia in a preclinical mouse model.”
Achondroplasia is the most common cause of dwarfism and is caused by mutation of the gene for fibroblast growth factor receptor 3 (FGFR3). FGFR3 has been shown to be crucial for the process of bone elongation.1,2 Infigratinib, an investigational agent, binds to FGFR3 and has been previously shown to increase bone growth in preclinical mouse models of achondroplasia.3 The data presented today showed a positive dose-response relationship between infigratinib and bone growth.
“Observing a dose-response relationship is an important step in developing infigratinib as a therapy for achondroplasia,” said Laurence Legeai-Mallet, Ph.D., head of research team at Imagine Institute, INSERM U1163, Université de Paris, who led the research. “We also observed better organization within the hypertrophic zone of the growth plate, which is responsible for bone formation and elongation, indicating that a low dose of infigratinib had a positive effect.”
The preclinical study used a dwarf mouse model (Fgfr3Y367C/+), which mimics achondroplasia. In each of the three dose groups, treatment with infigratinib led to an increase in all nine measures of bone size studied compared to the control group (See figure). At the highest dose examined (0.5 mg/kg/day), treatment with infigratinib demonstrated a statistically significant (P < 0.01) improvement in bone length of 7-14% in the upper limbs, 10% to 17% in the lower limbs and 12% in the foramen magnum (an opening at the base of the skull). No apparent toxicity of infigratinib was noted in this study.
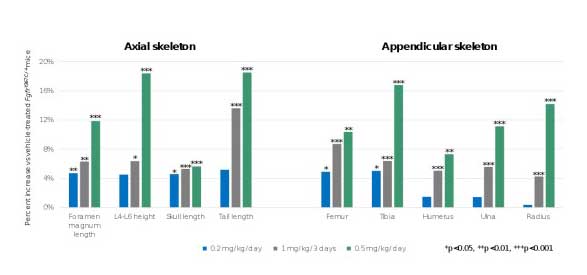
“We are encouraged by this preclinical data supporting the ability of a low dose, or even intermittently dosed, infigratinib to increase the length of the limbs as well as other bones in the skeleton. In particular, we believe the increase in foramen magnum size has significant implications, given that narrowing of this area of the skull can, in some cases, cause cervical spinal cord compression in achondroplastic children,” said Susan Moran, M.D., M.S.C.E., chief medical officer at QED. “These data support our decision to submit an investigational new drug application to the U.S. Food and Drug Administration and initiate a clinical trial in 2020 to investigate the potential to treat children with achondroplasia with infigratinib at doses 10 to 100 times lower than used in oncology indications.”
The full poster can be viewed at www.qedtx.com/ASHG2019/
About QED Therapeutics, Inc.
QED Therapeutics, a subsidiary of BridgeBio Pharma, is a biotechnology company focused on precision medicine for FGFR-driven diseases. Our lead investigational candidate is infigratinib (BGJ398), an orally administered, FGFR1-3 selective tyrosine kinase inhibitor that targets the protein responsible for many genetically-caused skeletal dysplasias. QED is also evaluating infigratinib in clinical studies for cancers driven by mutations in the FGFR1, 2, or 3 genes. We plan to conduct further clinical trials to evaluate the potential for infigratinib to treat people with other FGFR-driven tumor types and rare disorders.
For more information on QED Therapeutics, please visit the company’s website at www.qedtx.com
About BridgeBio Pharma, Inc.
BridgeBio is a team of experienced drug discoverers, developers and innovators working to create life-altering medicines that target well-characterized genetic diseases at their source. BridgeBio was founded in 2015 to identify and advance transformative medicines to treat patients who suffer from Mendelian diseases, which are diseases that arise from defects in a single gene, and cancers with clear genetic drivers. BridgeBio’s pipeline of over 15 development programs includes product candidates ranging from early discovery to late-stage development.
About the Imagine Institute:
As the first European center of research, care and education on genetic diseases, the Imagine Institute on the Necker-Enfants malades hospital AP-HP campus aims to understand them and cure them. The Institute brings together 1,000 of the best doctors, researchers and healthcare personnel in a creative architecture of synergies. It is this original continuum of expertise, combined with the proximity of patients, which allows Imagine to make discoveries to benefit the patients.
Since its creation, Imagine Institute has been supported by six founding Members : AP-HP, Inserm, Université Paris Descartes/Université de Paris, Fondation HP-HP, Mairie de Paris, AFM-Téléthon.
Some 8,000 identified genetic diseases affect 30 million patients in Europe, and nearly 3 million in France, where there are 30,000 new cases each year. Nearly 60% of children that have a consultation leave without a genetic diagnosis, and 90% of genetic diseases do not yet have a curative treatment. Faced with this major issue of public health, the challenge is two-fold: to diagnose and to cure.
BridgeBio Pharma Forward Looking Statements
This press release contains forward-looking statements. Statements we make in this press release may include statements which are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act), which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements, including statements relating to expectations, plans, and prospects regarding QED Therapeutics’ clinical development plans, clinical trial results, timing and completion of clinical trials, competitive environment, and the clinical and therapeutic potential of infigratinib for the treatment of achondroplasia, reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control including, without limitation, QED Therapeutics’ ability to initiate and continue its planned clinical trials of infigratinib, its ability to advance infigratinib in clinical development, including QED Therapeutics’ plans to file an IND in 2020, and the timing and success of any such continued clinical development, as well as those set forth in the Risk Factors section of BridgeBio Pharma, Inc.’s most recent Quarterly Report on Form 10-Q and BridgeBio’s other SEC filings. Moreover, QED Therapeutics operates in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of QED Therapeutics’ management as of the date of this release and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. All forward-looking statements in this press release are based on information available to us as of the date hereof, and except as required by law, we disclaim any obligation to update these forward-looking statements, whether as a result of new information, future events or otherwise.
_________________________
1Rousseau, F., Bonaventure, J., Legeai-Mallet, L., Pelet, A., Rozet, J.M., Maroteaux, P., Le Merrer, M., Munnich, A. “Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia”. Nature 371 (1994): 252–254.
2Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. “Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia.” Cell 78 (1994): 335–342.
3Komla-Ebri, D., Dambroise, E., Kramer, I., Benoist-Lasselin,C., Kaci, N., Le Gall, C., Martin, L., Busca, P., Barbault, F., Graus-Porta, D., Munnich, A., Kneissel, M., Di Rocco, F., Biosse-Duplan,M., Legeai-Mallet, L. “Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model”. J Clin Invest 126(5) (2016): 1871-84.
QED Contact:
Carolyn Hawley
Canale Communications
[email protected]
858-354-3581