BridgeBio Pharma, Inc. Reports First Quarter 2021 Financial Results And Business Update
–Received U.S. Food and Drug Administration (FDA) approval for NULIBRY™ (fosdenopterin) for injection as the first therapy to reduce the risk of mortality in patients with molybdenum cofactor deficiency (MoCD) Type A
–Reported proof-of-concept data of encaleret in Autosomal Dominant Hypocalcemia Type 1 (ADH1)
–Launched strategic collaboration with Helsinn Group to co-develop and commercialize infigratinib in oncology; BridgeBio eligible to receive payments up to approximately $2.45 billion USD
–Completed acquisition of Eidos Therapeutics, Inc.
–Raised nearly $750 million in gross proceeds through issuance of 2.25% Convertible Senior Notes due in 2029
–Ended quarter with $1,001.3 million in cash, cash equivalents and marketable securities
PALO ALTO, Calif., MAY 6, 2021 – BridgeBio Pharma, Inc. (Nasdaq: BBIO) (BridgeBio or the Company), a commercial-stage biopharmaceutical company founded to discover, create, test and deliver meaningful medicines for patients with genetic diseases and cancers with clear genetic drivers, today reported its financial results for the first quarter ended March 31, 2021 and provided an update on the Company’s operations.
“We measure success by the number of meaningful medicines we are able to develop and deliver to patients. Our first FDA approval in February was a significant milestone for us as a company, but more importantly marked a turning point for MoCD Type A patients and their families, who now have an approved therapy for the first time,” said BridgeBio CEO and founder Neil Kumar, Ph.D. “We hit the first of four major clinical data readouts anticipated over the next 12 months, reporting proof-of-concept data for encaleret in ADH1 that offers promise to patients in need. And we entered into a partnership with Helsinn Group, designed to help us reach as many patients as possible living with FGFR-driven cancers through our anticipated upcoming launch of infigratinib.”
Major milestones anticipated in 2021 or early 2022 for BridgeBio’s four core value drivers:
- Acoramidis (AG10) – Transthyretin (TTR) stabilizer for transthyretin amyloid cardiomyopathy (ATTR-CM): Topline results from Part A of the ATTRibute-CM trial are expected in late 2021 or early 2022 and from Part B in 2023. If Part A is successful, BridgeBio expects to submit an application for regulatory approval of acoramidis in 2022. ATTR is a form of amyloidosis caused by the accumulation of misfolded TTR protein. It is estimated to affect more than 400,000 people worldwide and is largely undiagnosed today.
- Encaleret – Calcium-sensing receptor (CaSR) inhibitor for ADH1: Early results from an ongoing Phase 2 proof-of-concept study shared at the Endocrine Society’s 2021 Annual Meeting (ENDO) in March 2021 showed normalization of blood calcium and urine calcium in 6 of 6 (100%) ADH1 participants. If the development program is successful, encaleret could be the first approved therapy for ADH1, a condition caused by gain of function variants in the CaSR gene estimated to be carried by 12,000 individuals in the United States alone.
- Low-dose infigratinib – FGFR1-3 inhibitor for achondroplasia: Initial data from the ongoing Phase 2 dose ranging study are expected by the end of 2021. Achondroplasia is the most common form of genetic short stature and one of the most common genetic diseases, with a prevalence of greater than 55,000 cases in the United States and European Union. Low-dose infigratinib is the only known product candidate in clinical development for achondroplasia that targets the disease at its genetic source and the only orally administered product candidate in clinical-stage development.
- BBP-631 – AAV5 gene therapy candidate for congenital adrenal hyperplasia (CAH): Investigational New Drug (IND) application cleared by the FDA and site activation for initiation of a first-in-human Phase 1/2 study is ongoing, with initial data anticipated in late 2021 or early 2022. CAH is one of the most prevalent genetic diseases potentially addressable with AAV gene therapy, with more than 75,000 cases estimated in the United States and European Union. The disease is caused by deleterious mutations in the gene encoding an enzyme called 21-hydroxylase, leading to lack of endogenous cortisol production. BridgeBio’s AAV5 gene therapy candidate is designed to provide a functional copy of the 21-hydroxylase-encoding gene (CYP21A2) and potentially address many aspects of the disease course.
Recent pipeline progress and corporate updates:
- Received FDA approval for NULIBRY™ (fosdenopterin) for injection in February 2021 as the first therapy to reduce the risk of mortality in patients with MoCD Type A, an ultra-rare, life-threatening genetic disorder that progresses rapidly, results in severe and largely irreversible neurological injury, and has a high infant mortality rate.
- Launched strategic collaboration with Helsinn Group in March 2021 to co-develop and commercialize infigratinib in oncology. The Company is eligible to receive up to approximately $2.45 billion USD, including over $100.0 million USD in upfront, regulatory and launch milestone payments, and the remainder subject to the achievement of specified commercial milestones, and retains full rights to infigratinib for use in skeletal dysplasias, including for achondroplasia.
- Completed acquisition of Eidos Therapeutics in January 2021, acquiring of all of the outstanding shares of Eidos common stock that BridgeBio did not already own.
- Raised nearly $750 million in gross proceeds in February 2021 through issuance of 2.25% Convertible Senior Notes due in 2029. The Company expects current cash, cash equivalents and marketable securities to support its planned operations into 2023.
- The Phase 3 clinical trial of topical patidegib gel in patients with Gorlin Syndrome, advanced by BridgeBio affiliate, PellePharm, failed to meet primary and secondary endpoints, but showed multiple signals of potential activity. Accordingly, the Phase 2 study in high-frequency basal cell carcinoma is being halted. The open-label extension (OLE) study is being continued at present at the behest of the patient foundations and physician leaders in the field. LEO Pharma, which entered into a strategic collaboration with PellePharm in 2018, has terminated its option to acquire PellePharm. PellePharm is currently evaluating its data and engaging the applicable regulatory authorities and potential strategic partners to determine next steps.
- Dosed first patient in Phase 2 trial of BBP-418 in Limb-Girdle Muscular Dystrophy Type 2i in February 2021.
- Dosed first healthy volunteer in Phase 1 trial of BBP-671, being developed for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and organic acidemias (OA), in April 2021.
- Announced formal partnerships in March 2021 with Brown University, University of California, San Diego, GlycoNet, The Lundquist Institute, Oregon Health & Science University, Roswell Park Comprehensive Cancer Center and University of California, Davis – for a total of 20 partnerships among BridgeBio and leading academic and research institutions to date.
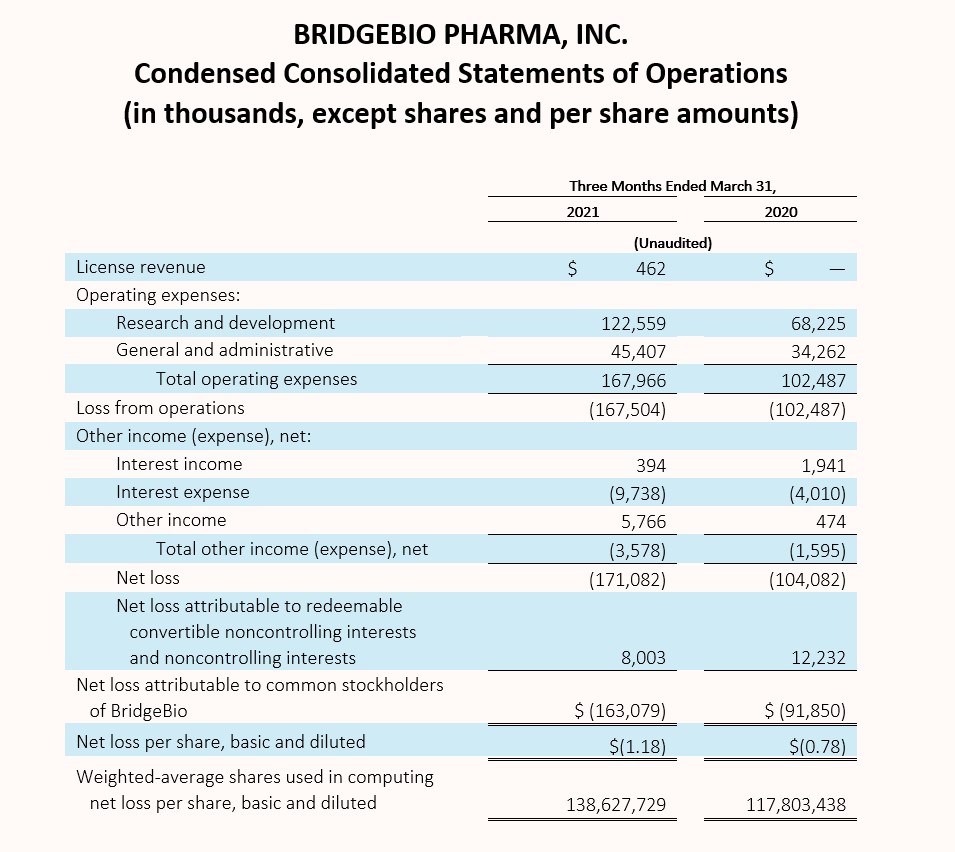
First Quarter 2021 Financial Results:
Cash, Cash Equivalents and Marketable Securities
Cash, cash equivalents and marketable securities, excluding restricted cash, totaled $1,001.3 million as of March 31, 2021, compared to $607.1 million as of December 31, 2020. The net increase in balance of $394.2 million is attributed to $731.4 million in net proceeds received from the issuance of our 2.25% Convertible Senior Notes due 2029 (2029 Notes), offset by a $61.3 million payment related to capped call options and a $50.0 million payment to repurchase shares of BridgeBio common stock, both in relation to the issuance of our 2029 Notes. In connection with our acquisition of Eidos, we also paid $59.1 million in direct transaction costs and $21.3 million to Eidos stockholders who elected cash settlement. The remaining change of $145.5 million primarily related to payments of interest and operating expenses.
Operating Expenses
Operating expenses for the three months ended March 31, 2021 were $168.0 million as compared to $102.5 million for the same period in the prior year. The increase in operating expenses of $65.5 million during the period is attributable to the increase in personnel costs resulting from an increase in the number of employees to support the progression in our research and development programs, including our increasing research pipelines, as well as an increase in stock-based compensation related to the achievement of various performance-based milestone compensation arrangements tied to regulatory and development milestones. Stock-based compensation for the three months ended March 31, 2021 was $34.9 million as compared to $10.2 million for the same period in the prior year.
Our research and development expenses have not been significantly impacted by the global outbreak of COVID-19 for the periods presented. While we experienced some delays in certain of our clinical enrollment and trial commencement activities, we continue to adapt in this unprecedented time to enable alternative site, telehealth and home visits, at-home drug delivery, as well as mitigation strategies with our contract manufacturing organizations. The longer-term impact, if any, of COVID-19 on our operating expenses is currently unknown.
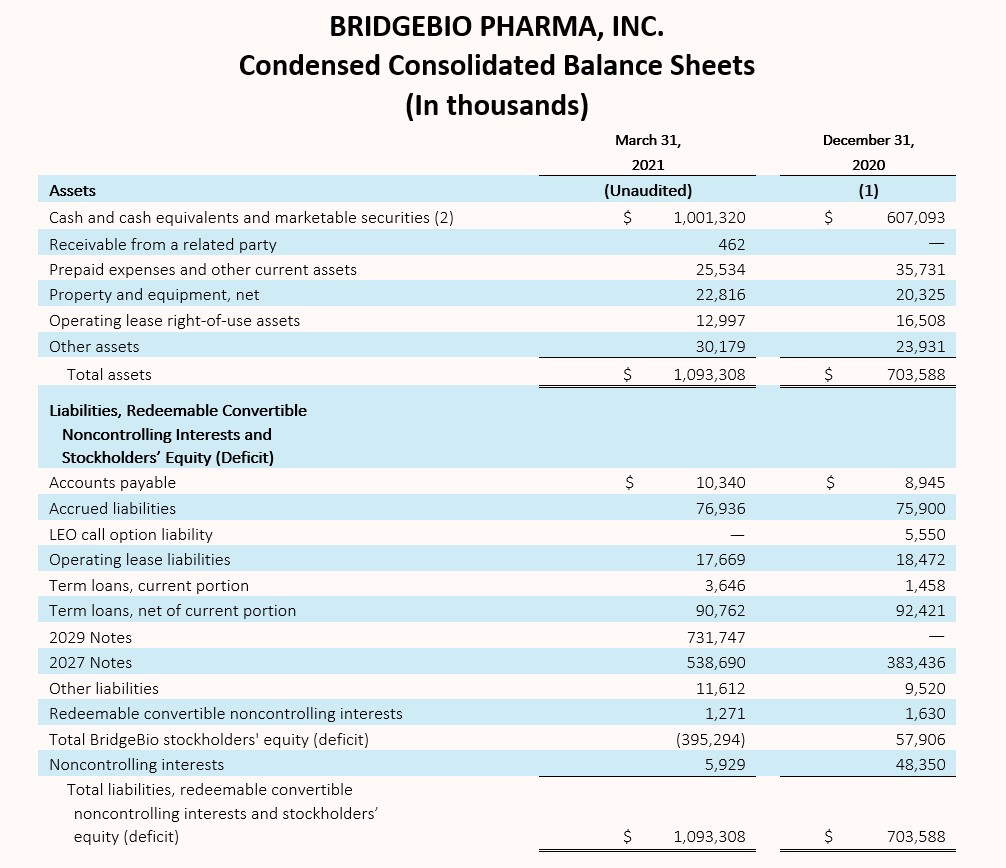
(2) March 31, 2021 amount includes long-term marketable securities of $82.2 million.
About BridgeBio Pharma
BridgeBio is a biopharmaceutical company founded to discover, create, test and deliver transformative medicines to treat patients who suffer from genetic diseases and cancers with clear genetic drivers. BridgeBio’s pipeline of over 30 development programs ranges from early science to advanced clinical trials and its commercial organization is focused on delivering the company’s first approved therapy. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit bridgebio.com.
Forward-Looking Statements
This press release contains forward-looking statements. Statements in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act. These forward-looking statements, including statements relating to the clinical and therapeutic potential of our programs and product candidates, the availability of topline results from Part A and Part B of our ATTRibute-CM trial of acoramidis, our plans to submit an application for regulatory approval of acoramidis, the availability of initial data from our ongoing Phase 2 study of infigratinib for achondroplasia and our ongoing Phase 1/2 study of BBP-631 for CAH, our eligibility to receive future milestone payments under our strategic collaboration with the Helsinn Group and the timing of these events, as well as our anticipated cash runway, reflect our current views about our plans, intentions, expectations and strategies, which are based on the information currently available to us and on assumptions we have made.
Although we believe that our plans, intentions, expectations and strategies as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a number of risks, uncertainties and assumptions, including, but not limited to, those risks set forth in the Risk Factors section of our Annual Report on Form 10-K for the year ended December 31, 2020, and our other filings with the U.S. Securities and Exchange Commission. Moreover, we operate in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of our management as of the date of this press release, and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Except as required by applicable law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
Contact:
Grace Rauh
BridgeBio Pharma, Inc.
[email protected]
(917) 232-5478
Source: BridgeBio Pharma, Inc.