SAN FRANCISCO, Sept. 05, 2019 (GLOBE NEWSWIRE) — BridgeBio Pharma, Inc. (Nasdaq: BBIO) subsidiary Origin Biosciences announced today the presentation of a study on the natural history of patients with Molybdenum Cofactor Deficiency (MoCD) and isolated sulfite oxidase deficiency (ISOD). The study was presented at the 2019 SSIEM Annual Symposium held in Rotterdam, Netherlands.1
In this comprehensive natural history study, 65 patients with MoCD and ISOD were enrolled from 27 participating centers across 14 countries. The majority of patients had MoCD Type A (n=37), followed by MoCD Type B (n=16) and Other (n=12). Data were collected retrospectively for all patients and prospectively for patients in the living cohort for up to one year. The primary endpoint was survival at 1 year of age.
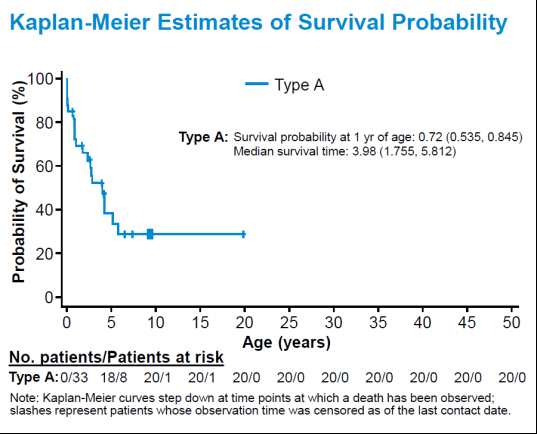
For MoCD Type A patients, the probability of survival at 1 year of age was 0.72 (0.535-0.845) and median survival was 3.98 years. Nearly all patients (n=57) had first presenting symptoms by Day 28 with median onset for MoCD Type A patients at 2 days. The most common initial symptoms were seizures and feeding difficulties. Other subsequent symptoms included developmental delay, truncal hypotonia with limb hypertonia, and cortical blindness. Median urinary S-sulfocysteine and xanthine concentrations were extremely high, and uric acid levels were drastically low in the first few days of life. Additional biochemical and neuroimaging data were collected. These data were presented during the Disorders of vitamins, cofactors and trace elements poster session at SSIEM. See below for a detailed summary of the data.
“Our findings confirm that the vast majority of MoCD and ISOD cases are severe, rapidly progressive, and neurodegenerative, resulting in significant disability and early lethality in almost all cases from the neonatal age,” said Ronen Spiegel, MD, Clinical Associate Professor, Director of Pediatric B Department, and Head of Metabolic Service, Emek Medical Center, principal investigator and lead author of the study.
Currently there are no approved therapies that alter the course of MoCD or ISOD. BridgeBio Pharma, through its subsidiary Origin Biosciences, is developing BBP-870 (ORGN001), a cPMP substrate replacement therapy that targets MoCD Type A directly at its source. By restoring the natural molybdenum cofactor synthetic pathway, the compound aims to reduce buildup of toxic sulfites and alleviate symptoms in infants and children with MoCD Type A. BBP-870 (ORGN001) has received Orphan Drug Designation in the US and Europe, Rare Pediatric Disease Designation and Breakthrough Therapy Designation from the FDA, and is currently being evaluated in a global Phase 2 clinical trial and a global Phase 2/3 clinical trial (NCT02629393; NCT02047461).
Key data from the natural history study:
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e270f0a3-80ee-4d0d-9e93-f0a7664270c4

About Molybdenum Cofactor Deficiency (MoCD)
MoCD is a rare, autosomal recessive, inborn error of metabolism caused by disruption in molybdenum cofactor (MoCo) synthesis that is vital for sulfite oxidase (SOX) activity. Patients are often infants with severe encephalopathy and intractable seizures. Disease progression is rapid with a high infant mortality rate.1,2 Those who survive beyond the first few months experience profuse developmental delays and suffer the effects of irreversible neurological damage, including brain atrophy with white matter necrosis, dysmorphic facial features, and spastic paraplegia.1,4 Clinical presentation that can be similar to hypoxic-ischemic encephalopathy (HIE) or other neonatal seizure disorders may lead to misdiagnosis and underdiagnosis.2,3 Immediate testing for elevated sulfite levels and S-sulfocysteine in the urine and very low serum uric acid may help with suspicion of MoCD.2,4
About Origin Biosciences
Origin Biosciences, a subsidiary of BridgeBio Pharma, is a biotechnology company focused on developing and commercializing a treatment for Molybdenum Cofactor Deficiency (MoCD) Type A. Origin is led by a team of veteran biotechnology executives. Together with patients and physicians, the company aims to bring a safe, effective treatment for MoCD Type A to market as quickly as possible.
For more information on Origin Biosciences, please visit the company’s website.
About BridgeBio Pharma
BridgeBio is a team of experienced drug discoverers, developers and innovators working to create life-altering medicines that target well-characterized genetic diseases at their source. BridgeBio was founded in 2015 to identify and advance transformative medicines to treat patients who suffer from Mendelian diseases, which are diseases that arise from defects in a single gene, and cancers with clear genetic drivers. BridgeBio’s pipeline of over 15 development programs includes product candidates ranging from early discovery to late-stage development.
BridgeBio Pharma Forward Looking Statements
This press release contains forward-looking statements. Statements we make in this press release may include statements which are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements, including statements relating to Origin Biosciences’ clinical development plans, including its plans to initiate a rolling NDA submission for BBP-870 (ORGN001), clinical trial results, timing and completion of clinical trials and regulatory submissions, competitive environment and clinical and therapeutic potential of BBP-870, reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control including, without limitation, Origin Biosciences’ ability to continue its planned clinical development and regulatory submissions for BBP-870 and the timing and success of any such continued clinical development and planned regulatory submissions, as well as those set forth in the Risk Factors section of BridgeBio Pharma Inc.’s most recent Quarterly Report on Form 10-Q and our other SEC filings. Except as required by law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
[1Spiegel R, Schwahn B, Scribner C L, Confer N. A natural history study of molybdenum cofactor (MoCo) and isolated sulfite oxidase deficiencies (ISOD). https://origintx.com/posters/
2Mechler K, Mountford WK, Hoffmann GF, Ries M. Ultra-orphan diseases: a quantitative analysis of the natural history of molybdenum cofactor deficiency. Genet Med. 2015;17(12):965-970.
3Durmaz MS, Özbakır B. Molybdenum cofactor deficiency: neuroimaging findings. Radiol Case Rep. 2018;13(3):592-595.
4Schwahn BC, Van Spronsen FJ, Belaidi AA, et al. Efficacy and safety of cyclic pyranopterin monophosphate substitution in severe molybdenum cofactor deficiency type A: a prospective cohort study. Lancet. 2015;386(10007):1955-1963.
Contacts
Alberto Gestri
AGestri@theoutcastagency.com