BBP-631
AAV5 gene therapy for congenital adrenal hyperplasia (CAH)
>75,000
CAH
CYP21A2
Phase 1
![]() gene therapy
gene therapy
BBP-631 is an investigational adeno-associated virus (AAV) gene therapy to treat CAH due to 21-hydroxylase deficiency at its source. BBP-631 is designed to deliver a functional copy of the 21-hydroxylase gene and has been shown through multiple animal studies to result in efficient and persistent delivery to the adrenal gland, where hormones are naturally made. If successful, we hope to restore the body’s hormone and steroid balance by enabling people with CAH to make their own cortisol and aldosterone — something that is not possible with any treatment on the market or currently in clinical trials for CAH. BridgeBio believes a gene therapy has the potential to restore the delicate balance of hormone production that is dysregulated in this disease.
CAH is a group of genetic disorders that affect the ability of the adrenal glands to produce many of the body’s crucial hormones.
The most common cause of CAH is a mutation in the gene encoding for 21-hydroxylase, an enzyme essential for making the hormones cortisol and aldosterone, which are critical for various physiologic functions. Cortisol is necessary for the body to respond to injury, stress or illness, and aldosterone is required to maintain proper blood pressure and sodium levels.
CAH has a severe form known as classic CAH and a milder form known as non-classic CAH. Classic CAH is a life-threatening disease that remains incapacitating with current treatment options and there is a need for truly effective therapies that have both strong short-term and long-term impact on the quality of life of the patients. A test for this inherited condition is included on every U.S. state’s newborn screening panel. Most individuals with classic CAH cannot produce cortisol, while patients with salt-wasting CAH (75% of classic CAH) additionally cannot produce sufficient aldosterone. Unable to produce these hormones, people with CAH cannot mount the healthy physiological response to stressors, such as illness, which can lead to an acute, life-threatening condition called adrenal crisis. These adrenal crises can be particularly dangerous for young children.
Moreover, deficiency of 21-hydroxylase results in an excess of hormones like testosterone and progesterone, also known as androgens. These excess androgens affect many aspects of growth and sexual development in both males and females throughout puberty. Continued dysregulation of androgens often leads to infertility in men and women and a variety of other issues.
Treatment of symptoms and risks of CAH are different in children versus in adults, but the mainstay of CAH treatment is lifelong daily glucocorticoids (e.g., steroids such as hydrocortisone, prednisone, and dexamethasone).
Lifelong steroid treatment can have significant side effects, and when coupled to the effects of the disease itself, patients face enormous challenges: Classic CAH patients have a lifespan 5 times shorter than age matched controls, and suffer from cardiovascular disease, metabolic disease, bone disease, and short stature (Mereke 2020, Gastaud et al., 2007). Additionally, both females and males struggle with significant infertility. Additionally, these treatments often fail to control excess androgen levels, leaving many symptoms of the disease unmanaged. Lastly, steroid supplementation does not recreate the normal daily rhythm of hormone production, which can lead to fatigue and sleep irregularities.
HPA Axis in CAH Due to 21-Hydroxylase Deficiency
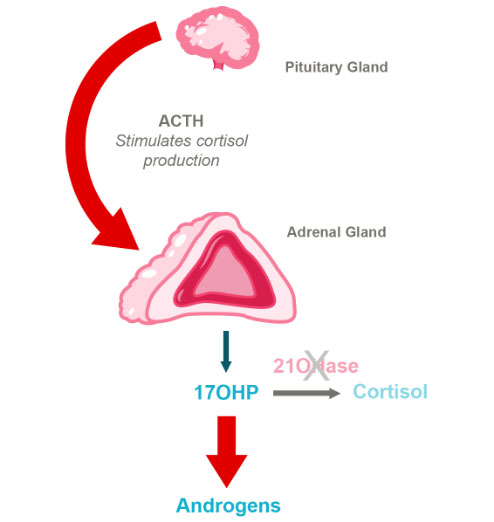
BBP-631 is designed to restore 21-hydroxylase
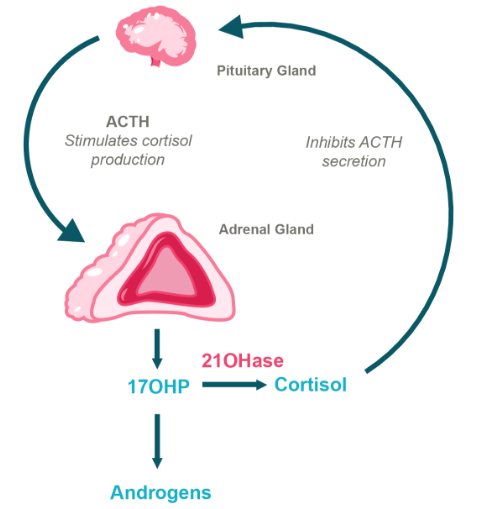
BridgeBio is developing BBP-631, an investigational AAV gene therapy, to treat the CAH due to 21-hydroxylase deficiency at its source. BBP-631 is designed to deliver a functional copy of the 21-hydroxylase gene to the adrenal gland.
If we are successful in expressing the 21-hydroxylase enzyme in the adrenal gland where hormones are naturally made, we hope to restore the body’s ability to self-regulate hormone production, which is not possible with any of the current treatment options. In sum, we believe a gene therapy has the potential to restore the delicate balance of hormone production that is dysregulated in this disease.
We have tested our CAH gene therapy in a mouse model with a mutation in the 21 hydroxylase gene. When untreated, the affected mice have poor weight gain and have severely elevated progesterone levels. Mice treated with gene therapy showed increased body weight and reduced progesterone levels, similar to normal mice.
Furthermore, we have performed testing in additional animal models, such as non-human primates, and have observed no significant safety findings in over 20 treated animals. Importantly, these animals show stable gene expression at least six months after treatment, indicating the potential for a durable treatment effect.
Every day, our work is inspired by patients and caregivers. Their partnership and collaboration is essential in understanding what’s meaningful and considering how BridgeBio can deliver medicines and services that fulfill the many unmet needs of patients. Learn more about our patient advocacy work by contacting [email protected].