Leadership
Eli Wallace, Ph.D. – CEO
Pedro Beltran, Ph.D. – CSO
Board of Directors
Frank McCormick, Ph.D. (Chair)
Neil Kumar, Ph.D.
Raymond Kelleher, M.D., Ph.D.
Michelle Doig
Our Programs
bbo-8520, a KRASG12C(ON) inhibitor
Phase 1 ONKORAS-101 trial currently enrolling
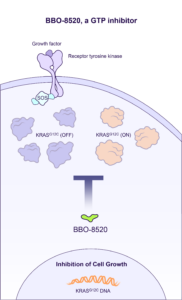
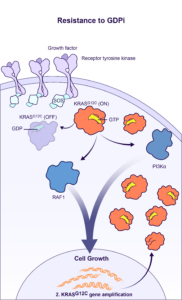
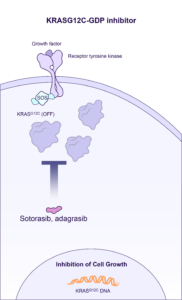
BBO-8520 is a direct inhibitor of KRASG12C that directly binds to the Switch II pocket in both the GTP-bound (ON) and GDP-bound (OFF) state conformations. BridgeBio Oncology Therapeutics is currently enrolling patients in the ONKORAS-101 trial for patients with KRASG12C mutant non-small cell lung cancer.
bbo-10203, a PI3Kα:RAS breaker
IND-enabling studies
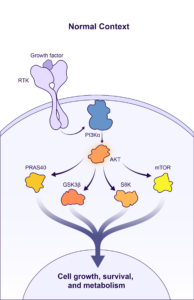
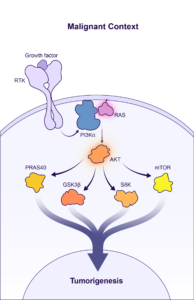
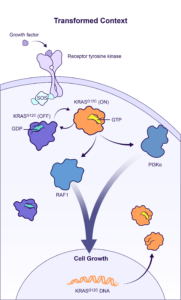
BBO-10203 is a PI3Kα:RAS breaker that blocks the specific interaction between RAS and PI3Kα to inhibit PI3Kα / AKT effector signaling in tumors while bypassing glucose metabolic signaling to avoid hyperglycemia. BridgeBio Oncology Therapeutics expects to file an Investigational New Drug (IND) application for BBO-10203 in Q2 2024, and subject to clearance of the IND, will begin enrolling patients later this year.

bbo-11818, a pan-KRAS inhibitor
IND-enabling studies
BBO-11818 is a pan-KRAS inhibitor that targets both the ON and OFF states of KRASG12X. BridgeBio Oncology Therapeutics expects to file an IND application for BBO-11818 in early 2025.